- #1
Awwtumn
- 47
- 3
https://en.wikipedia.org/wiki/Fluorescence
You normally heard of Fluorescence coming from UV which make the objects glow.
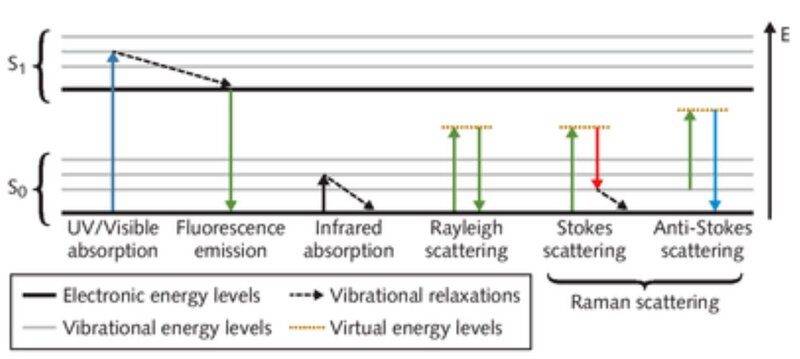
But visible light can also cause fluorescence. When you hit a colorful sample with a 532nm laser, there is fluorescence in the visible spectrum (as seen in Raman spectra). So how do you differentiate between the Fluorescence of objects that glow with UV absorption and visible light fluorescence and to that of visible light absorption and fluorescence? Specifically, do all color visible objects fluoresce? Whenever I aimed the 532nm Raman on color objects (such as red paint on a tin can), all I got are fluorescence which overlaps the small Raman signals.
Is this related to the fact that all colorful objects use paint in form of dyes and dyes can fluoresce? Can you give some list of colorful objects that won't fluoresce in visible light (using 532nm laser) so I can test this using Raman?
Also does this mean in everyday objects, there is continuous fluorescence from the visible light absorption and emission that we just don't see or distinguish with our naked eyes?
You normally heard of Fluorescence coming from UV which make the objects glow.
But visible light can also cause fluorescence. When you hit a colorful sample with a 532nm laser, there is fluorescence in the visible spectrum (as seen in Raman spectra). So how do you differentiate between the Fluorescence of objects that glow with UV absorption and visible light fluorescence and to that of visible light absorption and fluorescence? Specifically, do all color visible objects fluoresce? Whenever I aimed the 532nm Raman on color objects (such as red paint on a tin can), all I got are fluorescence which overlaps the small Raman signals.
Is this related to the fact that all colorful objects use paint in form of dyes and dyes can fluoresce? Can you give some list of colorful objects that won't fluoresce in visible light (using 532nm laser) so I can test this using Raman?
Also does this mean in everyday objects, there is continuous fluorescence from the visible light absorption and emission that we just don't see or distinguish with our naked eyes?