- #1
victor94
- 5
- 2
i'm trying to understand the solution to this problem:
http://physweb.bgu.ac.il/COURSES/StatMechCohen/ExercisesPool/EXERCISES/ex_2065_sol_Y13.pdf
(link to the problem and the solution of it)

All my questions come from the partition function:
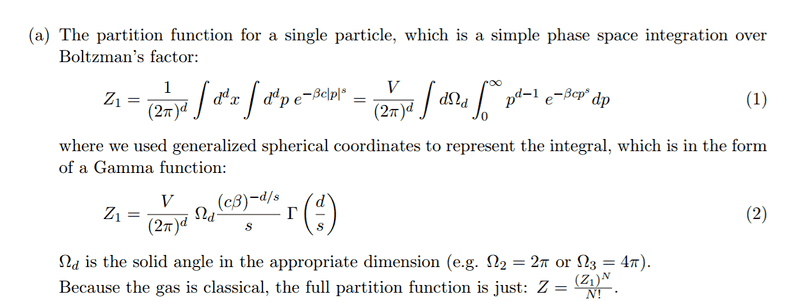
1) From where the term (2*pi)^d comes from?, I think is like a normalization factor, but I'm not sure.
2) The Volume (V) should be V^d, because is the volume of a particle of d dimension, but in the solution is just "V" , I don't understand why.3) The solid angle is used to simplify the integral and it comes from the volume of a sphere of d-dimensionm. I don't understand how to use that volume of the sphere to this specific problem.
4) Where is this problem used, or is it just a theoretical problem?
5) The last question is a conceptual one, how the phase space looks in a d-dimension, I don't understand this concept.
Any help in this questions will be appreciated.
Thanks in advance.
http://physweb.bgu.ac.il/COURSES/StatMechCohen/ExercisesPool/EXERCISES/ex_2065_sol_Y13.pdf
(link to the problem and the solution of it)
All my questions come from the partition function:
1) From where the term (2*pi)^d comes from?, I think is like a normalization factor, but I'm not sure.
2) The Volume (V) should be V^d, because is the volume of a particle of d dimension, but in the solution is just "V" , I don't understand why.3) The solid angle is used to simplify the integral and it comes from the volume of a sphere of d-dimensionm. I don't understand how to use that volume of the sphere to this specific problem.
4) Where is this problem used, or is it just a theoretical problem?
5) The last question is a conceptual one, how the phase space looks in a d-dimension, I don't understand this concept.
Any help in this questions will be appreciated.
Thanks in advance.
Attachments
Last edited: