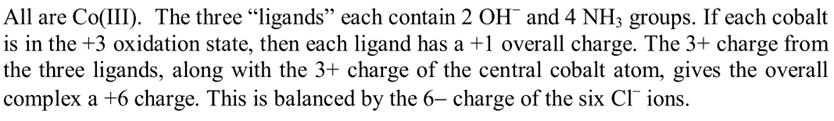Discussion Overview
The discussion revolves around the oxidation states of cobalt in a complex ion, with participants evaluating and critiquing various proposed oxidation states based on the coordination environment and theoretical considerations. The scope includes theoretical chemistry, practical applications, and conceptual clarifications regarding oxidation states in coordination complexes.
Discussion Character
- Debate/contested
- Conceptual clarification
- Technical explanation
Main Points Raised
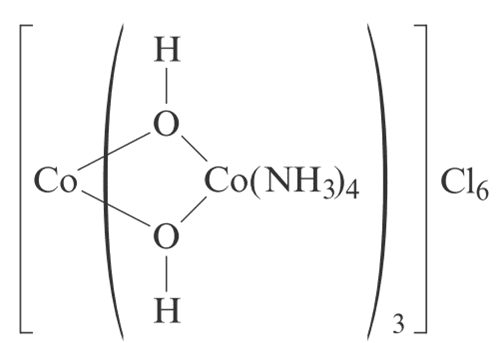
- One participant claims the oxidation state of central cobalt is +6 due to the presence of six oxygen ligands, while another cobalt is +2 due to two oxygen ligands and NH3, which does not contribute to the oxidation state.
- Another participant argues that oxidation states are merely an accounting device and do not reflect measurable properties of atoms, suggesting that assigning oxidation states in large complexes lacks real insight.
- A critique is raised regarding the total charge calculation based on the proposed oxidation states, questioning the balance with the number of chloride ions.
- One participant acknowledges missing hydroxide ions in their calculations, indicating a correction to their earlier statement.
- Another participant supports the utility of oxidation states in practical chemistry, citing examples where different oxidation states provide insight into the behavior of compounds.
- A later reply reiterates the argument against the existence of certain oxidation states, specifically stating that Co+6 is not a known oxidation state and questions the feasibility of such a state based on electron configuration.
Areas of Agreement / Disagreement
Participants express disagreement on the validity and utility of oxidation states, with some defending their use in practical contexts while others challenge their relevance in complex ions. The discussion remains unresolved regarding the correct oxidation states and their implications.
Contextual Notes
There are limitations in the discussion regarding the assumptions made about oxidation states, the definitions used, and the unresolved mathematical steps in charge balancing. The discussion also reflects differing perspectives on theoretical versus practical chemistry.