Ricky2357
- 23
- 0
1. The problem statement
Suppose we have a thermodynamical system whose state is modified by external forces. This results in a change of the internal energy of the system. If we designate by W_{ext} the total work done during the process by the external forces acting on the particles of the system, then the conservation of energy requires that
\Delta U=W_{ext}
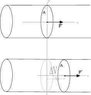
In the classic example of the container filled with gas whose volume can change by means
of a movable piston, the particles of the gas collide with the surface of the piston causing it to move by a small distance \Delta x (see figure attached).
Since no external forces act on this particular system we must have
W_{ext}=0 and thus \Delta U=0. But this can not be since the internal energy of the system clearly changes.
2. Relevant Questions
Where is the flaw in my thinking?
Suppose we have a thermodynamical system whose state is modified by external forces. This results in a change of the internal energy of the system. If we designate by W_{ext} the total work done during the process by the external forces acting on the particles of the system, then the conservation of energy requires that
\Delta U=W_{ext}
In the classic example of the container filled with gas whose volume can change by means
of a movable piston, the particles of the gas collide with the surface of the piston causing it to move by a small distance \Delta x (see figure attached).
Since no external forces act on this particular system we must have
W_{ext}=0 and thus \Delta U=0. But this can not be since the internal energy of the system clearly changes.
2. Relevant Questions
Where is the flaw in my thinking?