Dorslek
- 14
- 0
Let's start with a generalized example:
3A + 1B -> 2C
For the mole amount next to each molecule, am I always to consider these as relative to one another or absolute? Most of the videos I have seen are describing the above as "for every 3 moles of A and 1 mole of B you get 2 moles of C". I believed at some point that these numbers were absolute however, after reading up on equilibrium I discovered that I was incorrect.
So for a reaction such as this:
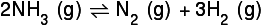
I have a ratio of 2:1:3. If I used up 0.8 moles of the nitrogen, I read that I would use the ratio to discover that I would produce 0.4 moles of nitrogen and 1.2 moles of hydrogen. Would anyone care to explain this to me?
3A + 1B -> 2C
For the mole amount next to each molecule, am I always to consider these as relative to one another or absolute? Most of the videos I have seen are describing the above as "for every 3 moles of A and 1 mole of B you get 2 moles of C". I believed at some point that these numbers were absolute however, after reading up on equilibrium I discovered that I was incorrect.
So for a reaction such as this:
I have a ratio of 2:1:3. If I used up 0.8 moles of the nitrogen, I read that I would use the ratio to discover that I would produce 0.4 moles of nitrogen and 1.2 moles of hydrogen. Would anyone care to explain this to me?