Cyclopse
- 10
- 0
Just to be sure, is this:
2-chloro,4-ethyl heptane ?
edit- i noticed i left out the hydrogens in the ethyl, pretend they r there :)
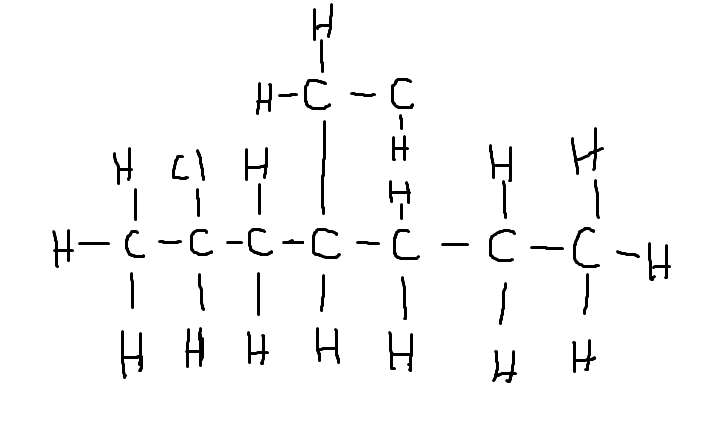
2-chloro,4-ethyl heptane ?
edit- i noticed i left out the hydrogens in the ethyl, pretend they r there :)