- #1
undertow
Why doesn't HOOH dipole dipole forces?
I read that if a molecule has an Oxygen atom, it's likely to have dipole forces. This one only has Dispersion forces.
Also, why does the Lewis structure of this molecule NOT look like this:
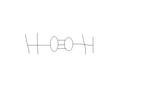
I read that if a molecule has an Oxygen atom, it's likely to have dipole forces. This one only has Dispersion forces.
Also, why does the Lewis structure of this molecule NOT look like this: