- #1
Wnt
- 1
- 0
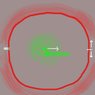
In a vacuum under standard gravity, an anchored spherical metal electrode is brought to +1,000,000 volts relative to the distant walls of a much larger chamber. (I'm a new user; hopefully this will display as attachment 1) Therefore, electrons that fall through a small gap in the electrode potentially emit kinetic energy greater than their 0.5 MeV rest mass. This energy comes from the electrostatic field that surrounds the spherical electrode (red haze; the energy in this field is proportional to the square of the magnitude of the force outward from the electrode, which is reduced by the negative charge of the electron inside). Within the sphere, there is no electrostatic field except that from the electron itself (green haze).
In this scenario, an electron is emitted from just outside the positive electrode so that it falls slowly through the center of the sphere, where it should experience no force from the shell of positive charge that surrounds it. The question is, as it crosses the chamber, will it strike ABOVE or BELOW the mark?
a) Gravity will pull the electrode downward according to its normal rest mass, like any particle; it is a curvature of space. As a consequence, it will force the center of mass of the electrostatic field in red to move upward.
b) Gravity will pull the electrostatic field downward, forcing the electron to move upward and strike above the mark.
And of course there's always c) you can tell me it's far, far more complicated than that. :)
In this scenario, an electron is emitted from just outside the positive electrode so that it falls slowly through the center of the sphere, where it should experience no force from the shell of positive charge that surrounds it. The question is, as it crosses the chamber, will it strike ABOVE or BELOW the mark?
a) Gravity will pull the electrode downward according to its normal rest mass, like any particle; it is a curvature of space. As a consequence, it will force the center of mass of the electrostatic field in red to move upward.
b) Gravity will pull the electrostatic field downward, forcing the electron to move upward and strike above the mark.
And of course there's always c) you can tell me it's far, far more complicated than that. :)