- #1
MathewsMD
- 433
- 7
Hi,
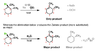
I'm just reviewing E1 and E2 mechanisms and I had one question regarding the catalysts. I've attached the image for the 2 reactions I have a question about.
I understand E1 mechanisms are more common for reactions with tertiary carbocation intermediates while E2 is more common for primary, and that E1 or E2 would occur for secondary (though it would depend on the intermediate stabilities, correct?). In the image posted, there are 2 reactions (E1 and E2) with the same original reagent. I just don't quite understand why OEt-, the catalyst for the E2 mechanism, cannot be used for the E1 mechanism (i.e. why is water used as opposed to OEt-?). They both seem to be LB, though OEt- stronger, and I would presume OEt- can be used as a catalyst for the E1 mechanism, but it appears not. Is there any particular reason why using OEt- (or any other molecule like OCH3-) does not lead to the E1 mechanism but instead to E2 only? Does it have to do with water being a smaller molecule and allowing the reaction to not be stereoselective? Why cannot the stronger LB like OEt- lead to the E1 mechanism?
I'm just reviewing E1 and E2 mechanisms and I had one question regarding the catalysts. I've attached the image for the 2 reactions I have a question about.
I understand E1 mechanisms are more common for reactions with tertiary carbocation intermediates while E2 is more common for primary, and that E1 or E2 would occur for secondary (though it would depend on the intermediate stabilities, correct?). In the image posted, there are 2 reactions (E1 and E2) with the same original reagent. I just don't quite understand why OEt-, the catalyst for the E2 mechanism, cannot be used for the E1 mechanism (i.e. why is water used as opposed to OEt-?). They both seem to be LB, though OEt- stronger, and I would presume OEt- can be used as a catalyst for the E1 mechanism, but it appears not. Is there any particular reason why using OEt- (or any other molecule like OCH3-) does not lead to the E1 mechanism but instead to E2 only? Does it have to do with water being a smaller molecule and allowing the reaction to not be stereoselective? Why cannot the stronger LB like OEt- lead to the E1 mechanism?