- #1
Tom Hardy
- 46
- 1
here is the question (part I):
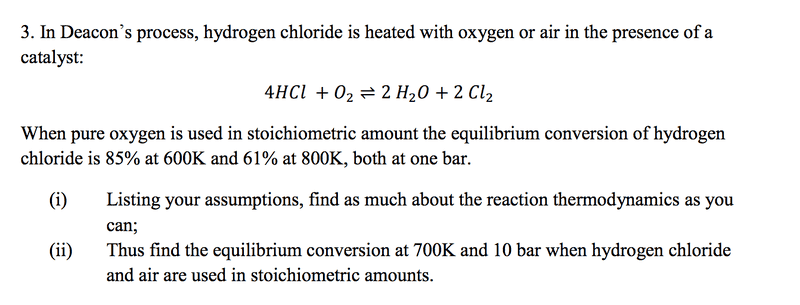 For part I, I need some assistance, I cannot figure out how to do the question. I know eventually what to do, it's just working out the equilibrium constants I'm having trouble with. So to start, I want to work out the K values at 600K and 800K, I do this by considering the total pressure:
For part I, I need some assistance, I cannot figure out how to do the question. I know eventually what to do, it's just working out the equilibrium constants I'm having trouble with. So to start, I want to work out the K values at 600K and 800K, I do this by considering the total pressure:
$$
\text{Total Pressure} = 1 \text{ bar}
$$
Initially:
$$
P^{o_2} + P^{HCl} = 1
$$
Since there are 4 mols of hcl per o2 we can write:
$$
5P^{o_2} = 1 \implies P^{O_2} = 0.2 \text{bar}
$$
85% conversion means that in the end, 85% of the oxygen has reacted therefore, there is 0.2*0.15 = 0.03 bar oxygen remaining, therefore 0.12 bar HCl remaining. In addition to this, each mol of oxygen reacted results in 4 new mols being created, therefore there are (0.85*0.2*2) bar of water (and also chlorine) produced, with this information, I can calculate my equilibrium constant, is this correct?
$$
\text{Total Pressure} = 1 \text{ bar}
$$
Initially:
$$
P^{o_2} + P^{HCl} = 1
$$
Since there are 4 mols of hcl per o2 we can write:
$$
5P^{o_2} = 1 \implies P^{O_2} = 0.2 \text{bar}
$$
85% conversion means that in the end, 85% of the oxygen has reacted therefore, there is 0.2*0.15 = 0.03 bar oxygen remaining, therefore 0.12 bar HCl remaining. In addition to this, each mol of oxygen reacted results in 4 new mols being created, therefore there are (0.85*0.2*2) bar of water (and also chlorine) produced, with this information, I can calculate my equilibrium constant, is this correct?