- #1
penguinnnnnx5
- 36
- 0
Homework Statement
The hydrogen molecular ion H1,2, with one electron and two protons, is the simplest molecule. The equilibrium spacing between the protons is 0.11 nm. Suppose the electron is at the midpoint between the protons and moving at 1.5 * 10^6 m/s perpendicular to a line between the protons.
How far (in nm) does the electron move before reaching a turning point? Because of their larger mass, the protons remain fixed during this interval of time.
Homework Equations
PE = kQq/r
KE = 1/2 mv2
W = KE + PE
k = 9*109
The Attempt at a Solution
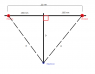
I first drew a diagram to get an idea of how to approach this problem.
Let x2 = .055 nm
R = √(D2 + x2)
-ΔKE = ΔPE
-(-KEi) = PEf
1/2 mv2 = kQq/R + kQq/R = 2kQq/R
R = 4kQq/(mv2) = √(D2 + x2)
D2 + x2 = [4kQq/(mv2)]2
D = √( [4kQq/(mv2)]2 - x2 )
D = ~.447 nm
This answer, however, is wrong. The correct answer is 3.0 * 10-3 nm.
I tried integrating from F, but I still found the same equation for PE. Am I doing something incorrect? Please help as I need to know this by tomorrow for my midterm. Thank you for any help received!