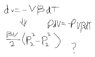geobot
- 3
- 0
Homework Statement
a) Estimate the pressure increase required to impart 1 J of mechanical work in reversibly compressing 1 mol of silver at room temperature.
b) What pressure rise would be required to impart 1 J of work to 1 mol of alumina at room temperature? For AL2O3 take the molar volume to be 25.715 (cc mol^-1) and Beta= 8.0 x 10^-7 (atm)^-1
Homework Equations
mechanical work = -PdV
dV=V.alpha.dT - V.beta.dP
(maybe: dU = (Cp - PV.alpha)dT + V(P.beta - T.alpha)dP )
The Attempt at a Solution
I have tried and tried to come up with a solution but I just can't figure it out.
See attached jpg.
I would appreciate any thoughts on the question
Attachments
Last edited: