Quentin_C
- 19
- 0
I was wondering how a boundary layer would be dissipative of momentum if it was under the influence of a positive heat gradient.
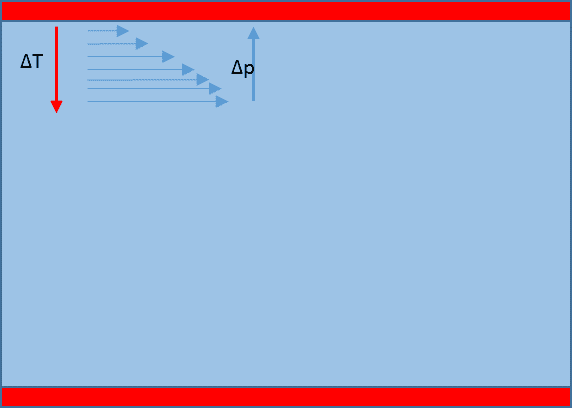
I understand that the reason that we don't see the boundary pressure equal the stagnation pressure is that the boundary is dissipative (so excess pressure above static pressure is "dumped" thru the pipe wall to the surroundings.)
If this heat gradient was reversed I could see that we would have an additional internal pressure gradient from a boundary quasi stagnation pressure to the lower static pressure in the moving fluid.
This gradient could be resolved by adding energy to the flowing fluid.
Should this mean that we would lose less momentum from the flow ?
I understand that the reason that we don't see the boundary pressure equal the stagnation pressure is that the boundary is dissipative (so excess pressure above static pressure is "dumped" thru the pipe wall to the surroundings.)
If this heat gradient was reversed I could see that we would have an additional internal pressure gradient from a boundary quasi stagnation pressure to the lower static pressure in the moving fluid.
This gradient could be resolved by adding energy to the flowing fluid.
Should this mean that we would lose less momentum from the flow ?