Air
- 202
- 0
Homework Statement
With the 5 equations, the equilibrium contants can be calculated at the bottom. (See image)
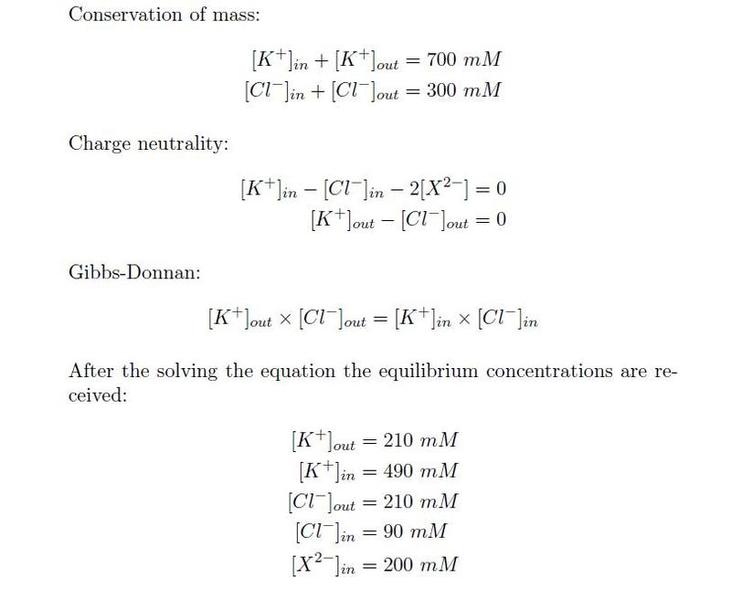
My complication
I am aware that X = 200 thus that value remains at that. Also, From the fourth equation K_{out} = Cl_{out}. But, I cannot seem to work out the values. If not simple algebra, what am I missing?
With the 5 equations, the equilibrium contants can be calculated at the bottom. (See image)
My complication
I am aware that X = 200 thus that value remains at that. Also, From the fourth equation K_{out} = Cl_{out}. But, I cannot seem to work out the values. If not simple algebra, what am I missing?