Spruance
- 33
- 0
The diagram shows some of the energy levels of an electron in a mercury atom. Level Q represents the lowest possible energy level.
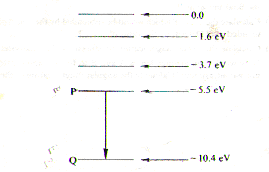
(a)Explain why a line spectrum results from an atom with such energy levels.
(b) Calculate the energy change in joules when the electron moves from level P to level Q and determine the wavelength of the spectral line associated with this transition.
(c)Explain what is likely to happen if a moving electron of energy 7.0eV collides with an isolated mercury atom in the ground state.
(d)Explain what is likely to happen if a photon, also of energy 7.0eV were to be incident on the atom.
Planck’s constant = 6.6 x 10-34 Js,
speed of light = 3.0 x 108m/s,
charge on an electron = 1.6 x 10 -19 C
Thanks in advance
(a)Explain why a line spectrum results from an atom with such energy levels.
(b) Calculate the energy change in joules when the electron moves from level P to level Q and determine the wavelength of the spectral line associated with this transition.
(c)Explain what is likely to happen if a moving electron of energy 7.0eV collides with an isolated mercury atom in the ground state.
(d)Explain what is likely to happen if a photon, also of energy 7.0eV were to be incident on the atom.
Planck’s constant = 6.6 x 10-34 Js,
speed of light = 3.0 x 108m/s,
charge on an electron = 1.6 x 10 -19 C
Thanks in advance