stratz
- 23
- 0
Edit by Mentor: thread moved from the other forum, hence no template used.
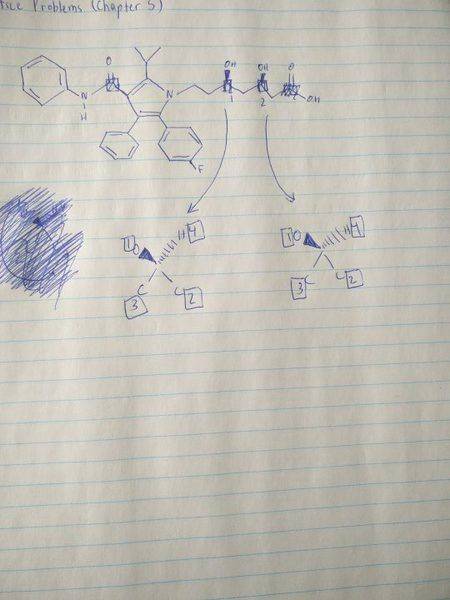
The problem tells me to determine the S/R configurations of each chiral center in the compound, which I have drawn in the picture above. As you can see the numbers assigned by atomic # are not in order. How would I go about determining configuration in this case?
The problem tells me to determine the S/R configurations of each chiral center in the compound, which I have drawn in the picture above. As you can see the numbers assigned by atomic # are not in order. How would I go about determining configuration in this case?