- #1
Jed K
- 3
- 0
So I understand generally how humidity in atmospherics work but there's this one thing I can't reconcile with the fundamentals.
First, my understanding of the basics. Water boils at 212 F @ 14.7 psia (1 ATM). At 213F @ 14.7 psia that water is now 1F superheated vapor. So we're all on the same page the boiling point here is also referred to the "bubble point" (i.e. the liquid to saturated liquid-vapor transition line). The dew point as discussed in normal atmospheric terminology is the point at which the water vapor (superheated vapor) starts to condense out (i.e. the vapor to saturated liquid-vapor transition line).
Ok. The thing I can't reconcile is under "normal" atmospheric conditions, how is there superheated water vapor in the air? My first intuition was that as altitude increases the pressure decreases and therefore so does the bubble point, but doing some rough calculations to convert standard atmosphere to a pressure at a given altitude where clouds start to form (~6500 ft), it doesn't seem like the pressure by itself drops enough to lead to evaporation. Additionally, as the altitude increases the temperate decreases so it seems to me the decreasing pressure and temperature cancels each other out, at least to some extent. Lastly, evaporation has to happen at ground level around bodies of water to begin with.
So, how is evaporation even occurring under normal atmospheric conditions (i.e. around 14.7 psia and way, way below 212F).
Below I'm including a ph-diagram of water for reference.
Any help would be greatly appreciated!
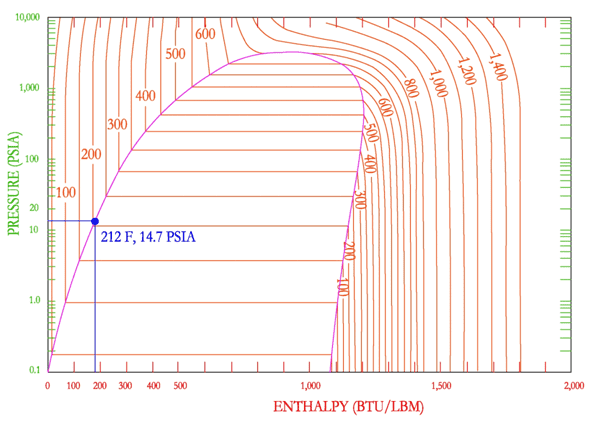
First, my understanding of the basics. Water boils at 212 F @ 14.7 psia (1 ATM). At 213F @ 14.7 psia that water is now 1F superheated vapor. So we're all on the same page the boiling point here is also referred to the "bubble point" (i.e. the liquid to saturated liquid-vapor transition line). The dew point as discussed in normal atmospheric terminology is the point at which the water vapor (superheated vapor) starts to condense out (i.e. the vapor to saturated liquid-vapor transition line).
Ok. The thing I can't reconcile is under "normal" atmospheric conditions, how is there superheated water vapor in the air? My first intuition was that as altitude increases the pressure decreases and therefore so does the bubble point, but doing some rough calculations to convert standard atmosphere to a pressure at a given altitude where clouds start to form (~6500 ft), it doesn't seem like the pressure by itself drops enough to lead to evaporation. Additionally, as the altitude increases the temperate decreases so it seems to me the decreasing pressure and temperature cancels each other out, at least to some extent. Lastly, evaporation has to happen at ground level around bodies of water to begin with.
So, how is evaporation even occurring under normal atmospheric conditions (i.e. around 14.7 psia and way, way below 212F).
Below I'm including a ph-diagram of water for reference.
Any help would be greatly appreciated!
