Discussion Overview
The discussion revolves around determining the number of peaks in the NMR spectrum of Malic Acid (HOOC CH2 CH(OH) COOH) when using a solvent other than D2O. Participants explore the implications of different hydrogen environments in the molecule and how these relate to the peaks observed in the NMR spectrum.
Discussion Character
- Homework-related
- Exploratory
- Technical explanation
- Debate/contested
Main Points Raised
- Some participants suggest that the two -COOH groups will have identical hydrogen environments, leading to one peak for both, while others propose that there may be a total of 4 or 5 peaks depending on the identity of the carboxylic acid groups.
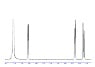
- A participant asks for an example of the NMR graph and inquires about identifying the number of peaks and their integration.
- Another participant mentions that databases like SDBS contain spectra of malic acid in aprotic solvents, which align with basic NMR theory.
- One participant expresses difficulty in analyzing the H-NMR spectrum of Malic Acid and questions how to determine its structure from the graph.
- There is a suggestion that each proton in malic acid could represent different environments, potentially leading to 6 distinct peaks.
- Participants discuss the concept of integration in NMR and how it relates to the number of protons corresponding to each peak.
Areas of Agreement / Disagreement
There is no clear consensus on the exact number of peaks in the NMR spectrum of Malic Acid, as participants present differing views on the hydrogen environments. The discussion remains unresolved regarding the specifics of peak identification and integration.
Contextual Notes
Participants express uncertainty about the integration process and how it applies to the specific NMR graph of Malic Acid. The discussion includes references to external databases for spectral data, but limitations in understanding the integration and peak analysis remain.
Who May Find This Useful
This discussion may be useful for students and researchers interested in NMR spectroscopy, particularly those studying organic compounds like Malic Acid and seeking to understand peak analysis and integration in spectral data.