You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How many Rb atoms to fit on a 9Cm^2 square?
AI Thread Summary
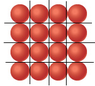
The discussion centers on calculating how many rubidium (Rb) atoms can fit on a 9 cm² square. Participants explore the atomic radius of Rb, which is approximately 2.475 x 10^-8 cm, and discuss the need to account for empty spaces between atoms in their calculations. There is confusion regarding the geometric arrangement of atoms, with suggestions to use parallelograms instead of triangles for more accurate area calculations. The conversation also touches on the need for visual aids, with requests for diagrams to clarify the concepts. Ultimately, one participant reports successfully solving the problem after extensive deliberation.
Physics news on Phys.org
mfb
Mentor
- 37,371
- 14,197
There are empty spaces between the atoms - they need more space than just pi*r2.
UnD3R0aTh
- 90
- 0
exactly my thought, how to calculate that?!
mfb
Mentor
- 37,371
- 14,197
UnD3R0aTh
- 90
- 0
excellent, i solved the first one, but the second one I'm not really sure how to calculate the side of the triangle that's enclosing one atom!
mfb
Mentor
- 37,371
- 14,197
You can draw an equilateral triangle in the lattice.
UnD3R0aTh
- 90
- 0
yes but it looks like it's side is longer than the diameter, how to calculate it's length?!
UnD3R0aTh
- 90
- 0
no wait, the triangles do not work, they overlap! parallelograms work! how to calculate the area of a parallelogram using it's sides?
mfb
Mentor
- 37,371
- 14,197
Then you are drawing the wrong triangles. Use the centers of the atoms, this is easier. Afterwards, find out how many atoms per triangle (or triangles per atom) you have.
UnD3R0aTh
- 90
- 0
can u please draw a diagram like u did with the first one?
mfb
Mentor
- 37,371
- 14,197
Can you show your diagram?
UnD3R0aTh
- 90
- 0
i don't know to how to draw this, using what program?
mfb
Mentor
- 37,371
- 14,197
Every drawing program can draw lines. Windows always comes with Paint, for example.
UnD3R0aTh
- 90
- 0
pffffft I've been thinking about this problem for far too long I'm at my wits end, i really need to stop wasting my time with this and study, i feel nervous already cause i wasted so much time, please give me a more strong hint, save me some time, thank you
UnD3R0aTh
- 90
- 0
solved it
Similar threads
- Replies
- 1
- Views
- 2K
- Replies
- 11
- Views
- 17K
- Replies
- 3
- Views
- 2K
- Replies
- 2
- Views
- 11K
- Replies
- 1
- Views
- 637
- Replies
- 18
- Views
- 2K
- Replies
- 1
- Views
- 3K
- Replies
- 2
- Views
- 2K
- Replies
- 1
- Views
- 1K
- Replies
- 8
- Views
- 1K
Hot Threads
-
Confusion regarding a chemical kinetics problem
- Started by palaphys
- Replies: 12
- Biology and Chemistry Homework Help
-
Biology Prove that evolution is constant (Provide at least two arguments to support your position)
- Started by yo yo
- Replies: 24
- Biology and Chemistry Homework Help
Recent Insights
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 7
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 0
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 0
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 78
- General Math
-
Insights Why Vector Spaces Explain The World: A Historical Perspective
- Started by fresh_42
- Replies: 0
- General Math