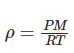Ideal gas law in terms of density
Click For Summary
SUMMARY
The discussion focuses on applying the Ideal Gas Law (PV=nRT) to derive pressure as a function of height using density. The participants explore the integration of the equation -ρg = -PMg/RT = dP/dy, emphasizing the need for understanding integration techniques. The conversation highlights the importance of recognizing n as the number of moles, defined as mass divided by molecular mass. The final goal is to evaluate pressure at a specific height (8812 m) using definite integration.
PREREQUISITES- Understanding of the Ideal Gas Law (PV=nRT)
- Basic knowledge of calculus, specifically integration and differentiation
- Familiarity with physical concepts of pressure and density
- Knowledge of constants such as gravitational acceleration (g) and gas constant (R)
- Learn integration techniques, particularly definite and indefinite integrals
- Study the relationship between pressure, density, and height in fluid mechanics
- Explore the application of the Ideal Gas Law in various thermodynamic processes
- Investigate the significance of molecular mass in gas calculations
Students studying physics or chemistry, particularly those focusing on thermodynamics and fluid mechanics, as well as educators seeking to clarify concepts related to the Ideal Gas Law and integration techniques.
Similar threads
- · Replies 9 ·
- · Replies 2 ·
- · Replies 3 ·
- · Replies 2 ·
- · Replies 3 ·
- · Replies 3 ·
- · Replies 2 ·
- · Replies 12 ·