SUMMARY
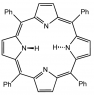
Tetraphenylporphyrin (TPP) is determined to be nonpolar due to its symmetrical structure, which allows dipoles from the N-H bonds to cancel each other out. The presence of a center of symmetry in TPP means that any dipole created would be negated, resulting in an overall nonpolar molecule. However, when TPP reacts with copper (II) acetate, forming a Copper TPP complex, the introduction of the copper (II) ion alters the polarity, making the complex polar due to the electron distribution influenced by the copper ion's electronic configuration of [Ar] 3d9.
PREREQUISITES
- Understanding of molecular symmetry and dipole moments
- Knowledge of coordination chemistry and metal-ligand interactions
- Familiarity with the electronic configuration of transition metals
- Basic principles of acid-base reactions involving acetate
NEXT STEPS
- Study molecular symmetry and its effects on polarity in organic compounds
- Explore coordination chemistry, focusing on the behavior of transition metal complexes
- Learn about the electronic configurations of transition metals and their impact on molecular properties
- Investigate acid-base reactions involving metal acetates and their resulting complexes
USEFUL FOR
Chemistry students, organic chemists, and researchers interested in molecular polarity and coordination complexes.