- #1
requied
- 98
- 3
Summary:: How to load the plates with the Lead element in the spectrometer
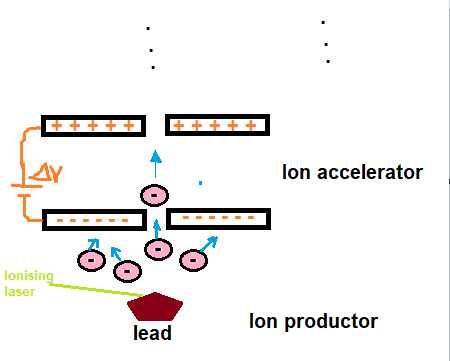
I have a mass spectrometer with lead element which has an electronic configuration 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^2 6p^2. It has 2 free electron, so the ejected electrons go through the ion accelerator and here, to speed the electrons I thougth the plates' charges must be like that (above must be +, below must be -). It's right or not?
And also I have a table like :
I have a statement like "The ionized isotopes are in Pb^- form. That is only a single electron is missing in lead ions. The number of ionized isotopes entering into the accelerator region corresponds to a 1μA ion current.". What does it mean that Pb^- form and 1μA? I also wondering that which isotopes will I use to find Vacc, Eacc etc.
Note: If this thread resides question threads, please transport there. I don't start this there because of I wasn't sure about. Please don't delete the thread.
[Moderator's note: Moved from a technical forum and thus no template.]
I have a mass spectrometer with lead element which has an electronic configuration 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^2 6p^2. It has 2 free electron, so the ejected electrons go through the ion accelerator and here, to speed the electrons I thougth the plates' charges must be like that (above must be +, below must be -). It's right or not?
And also I have a table like :
| Lead Isotopes | Relative Abudance | Decays | Isotope Mass(AMU) |
| Pb204 | 1.4% | Stable | 203.978 |
| Pb206 | 24.1% | Stable | 205.974 |
| Pb207 | 22.1% | Stable | 206.975 |
| Pb208 | 52.4% | Stable | 207.976 |
| Lead | 100% | 207.2 |
I have a statement like "The ionized isotopes are in Pb^- form. That is only a single electron is missing in lead ions. The number of ionized isotopes entering into the accelerator region corresponds to a 1μA ion current.". What does it mean that Pb^- form and 1μA? I also wondering that which isotopes will I use to find Vacc, Eacc etc.
Note: If this thread resides question threads, please transport there. I don't start this there because of I wasn't sure about. Please don't delete the thread.
[Moderator's note: Moved from a technical forum and thus no template.]
Last edited by a moderator: