- #1
Aly_19f
- 9
- 1
- TL;DR Summary
- How do I calculate the number density of each element in an enriched fuel?
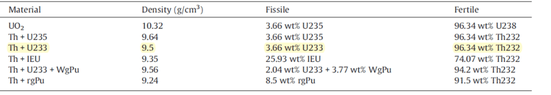
Hey there, I'm working on an MCNPX modelling for SCWR using different clads and fuels, the first fuel was UO2(5%) and I have calculated the number density correctly since there was only one vector U.
But now I don't know how top deal with the Th+U233 due to the existence of Thorium.
Can anyone help me or guide me to a similar solved problem (Uranium + another heavy fuel)?
But now I don't know how top deal with the Th+U233 due to the existence of Thorium.
Can anyone help me or guide me to a similar solved problem (Uranium + another heavy fuel)?