bomba923
- 759
- 0
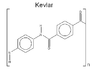
(According to Wikipedia, the strongest hydrogen bonds are O—H...N bonds, occurring between a hydroxyl's hydrogen and a nearby nitrogen atom. That's when I thought of Kevlar...)[/size]
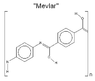
Can Mevlar* be stronger than Kevlar? (see the attached images)
(*My slight modification of the Kevlar monomer )
)
Can Mevlar* be stronger than Kevlar? (see the attached images)
(*My slight modification of the Kevlar monomer
Attachments
Last edited: