Organic chemistry acetal reaction help
- Thread starter IntegralDerivative
- Start date
Click For Summary
Discussion Overview
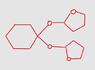
The discussion revolves around the acetal formation reaction in organic chemistry, specifically focusing on the role of cyclohexanol and the necessary functional groups involved in the reaction. Participants explore the steps leading to acetal formation, including the intermediate hemiacetal, and clarify the conditions required for the reaction.
Discussion Character
- Technical explanation
- Homework-related
- Exploratory
Main Points Raised
- Some participants question the reasoning behind cyclohexanol hosting an acetal carbon and whether the discussion pertains to homework.
- There is a discussion about the necessary functional groups for acetal formation, with mentions of alcohols, leaving groups, and acid catalysts.
- Participants discuss the formation of a hemiacetal when one equivalent of alcohol is added to a carbonyl group, with some uncertainty about the need for oxidizing agents to form carbonyls.
- Clarifications are made regarding the steps involved in the reaction, including the distinction between hemiacetals and oxonium ions.
- One participant expresses confusion about the naming of the resulting molecule and the steps involved in the reaction.
Areas of Agreement / Disagreement
Participants generally agree on the formation of a hemiacetal as an intermediate step, but there is uncertainty regarding the specific functionalities involved and the overall reaction pathway. The discussion remains unresolved on some points, particularly concerning the necessity of oxidizing agents and the naming of the final product.
Contextual Notes
Some participants express confusion about the steps in the reaction and the definitions of terms like hemiacetal and oxonium, indicating potential gaps in understanding the underlying chemistry.
Similar threads
- · Replies 2 ·
- · Replies 28 ·
- · Replies 7 ·
- · Replies 6 ·
- · Replies 4 ·
- · Replies 8 ·
- · Replies 4 ·
- · Replies 2 ·
- · Replies 3 ·