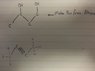
SUMMARY
The discussion focuses on synthesizing alcohol from an alkyne using hydration reactions and specific reagents. The suggested method involves converting the alkyne to an alkene using sodium in liquid ammonia, followed by bromination with N-Bromosuccinimide (NBS) to produce allyl bromide. The reaction with aqueous sodium hydroxide yields the desired alcohol, which can then undergo acidic hydrolysis to form 1,3-butanediol. Key corrections were made regarding the structure of the alkyne and the reaction pathway.
PREREQUISITES
- Understanding of organic chemistry principles, specifically hydration reactions.
- Familiarity with alkyne and alkene structures and their hybridization.
- Knowledge of reagents such as N-Bromosuccinimide (NBS) and sodium in liquid ammonia.
- Experience with substitution reactions and acidic hydrolysis in organic synthesis.
NEXT STEPS
- Research the mechanism of hydration reactions involving alkynes and alkenes.
- Learn about the use of N-Bromosuccinimide (NBS) in organic synthesis.
- Study the process of acidic hydrolysis and its applications in alcohol synthesis.
- Explore the synthesis of 1,3-butanediol and its industrial applications.
USEFUL FOR
Organic chemists, students studying organic synthesis, and anyone involved in alcohol production from alkynes will benefit from this discussion.