JDoolin
Gold Member
- 723
- 9
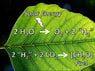
CO2 appears to create global warming, but is there a way to get rid of the CO2? Could we somehow industrialize the photosynthesis process to get rid of large amounts of CO2?
What sorts of issues prevent this solution?
What sorts of issues prevent this solution?