ptpatil
- 6
- 0
Phthalic Anhydride derived Polyesters and Nylon structures+reactions?
Im doing a lab for Materials science for engineers.
they want to know how phthalic anhydride and ethylene glycol makes a polyester. They also want to know how that and glycerin make a polyester.
After that we made nylon from sebacoyl chloride and hexamethylenediamine.
Im a first year student and I have no clue how these reactions work, they didnt teach us about the chemistry just the mechanical properties once the polymer chains are formed.
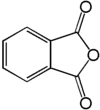
Phtalic anhydride:
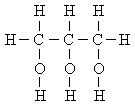
ethylene glycol:glycerin:
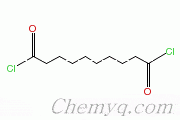
sebacoyl chloride:
hexamethylenediamine:
I figured that the OHs would probably react with each other on the ends of the molecule but I have no idea what the polyester structure would be of the either the phthalic anhydride derived polyesters or the nylon-6,10.
I do know that there are probably two reactions from the ethylene glycol with the oxygens in the phthalic anhydride but nothing further than that.
Homework Statement
Im doing a lab for Materials science for engineers.
they want to know how phthalic anhydride and ethylene glycol makes a polyester. They also want to know how that and glycerin make a polyester.
After that we made nylon from sebacoyl chloride and hexamethylenediamine.
Im a first year student and I have no clue how these reactions work, they didnt teach us about the chemistry just the mechanical properties once the polymer chains are formed.
Homework Equations
Phtalic anhydride:
ethylene glycol:glycerin:
sebacoyl chloride:
hexamethylenediamine:
The Attempt at a Solution
I figured that the OHs would probably react with each other on the ends of the molecule but I have no idea what the polyester structure would be of the either the phthalic anhydride derived polyesters or the nylon-6,10.
I do know that there are probably two reactions from the ethylene glycol with the oxygens in the phthalic anhydride but nothing further than that.
Last edited by a moderator: