- #1
- 2,116
- 2,691
I could finally get a copy of the Red Book 2005 from a library. In the chapter dedicated to nomenclature of coordinate complexes, while explaining the κ-system of specifying donor atoms, the following two examples were given (among many others):
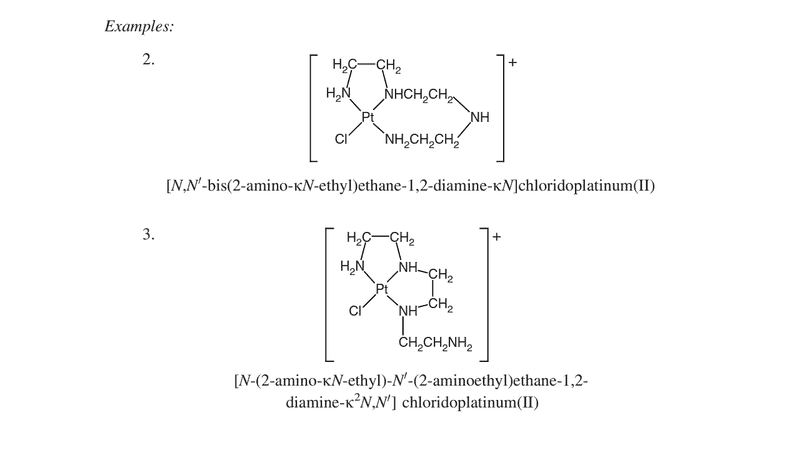
Can anyone explain how the naming is done for the two compounds (labelled 2 and 3 in the picture)? The problem that I am facing is with the closed rings. From where is the naming started?
Can anyone explain how the naming is done for the two compounds (labelled 2 and 3 in the picture)? The problem that I am facing is with the closed rings. From where is the naming started?
