- #1
JHUK
- 5
- 0
I posted in picture format to post on another website, but haven't found a reply yet:
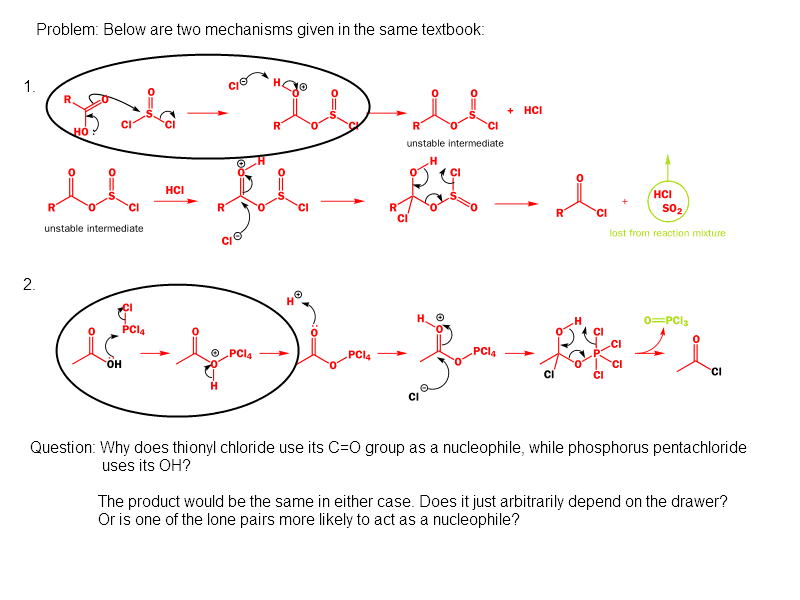
Yanick said:You're question is a little unclear because the mechanism shows nucleophilic attack by a -COOH group. The oxygens in these groups are actually equivalent and are best represented by resonance structures where the hydroxyl and carbonyl oxygens exchange.
sjb-2812 said:No, they are equivalent in the carboxylate anion, but not in the protonated version. Resonance structures do not involve the movement of protons.
Oxygen can act as a nucleophile by donating a lone pair of electrons to a positively charged or electron deficient atom, forming a covalent bond. This allows oxygen to participate in chemical reactions and bond with other atoms.
A nucleophile is an atom or molecule that has a high electron density and is able to donate a pair of electrons to form a new covalent bond. Nucleophiles are commonly involved in chemical reactions and can bond with electrophiles, which are atoms or molecules with a low electron density.
Oxygen's electronegativity, or its ability to attract electrons, makes it a strong nucleophile. This is because oxygen's high electronegativity causes it to have a high electron density, making it more likely to donate a pair of electrons to form a covalent bond.
Yes, oxygen can act as a nucleophile in both polar and nonpolar solvents. In polar solvents, oxygen's high electronegativity allows it to interact with other polar molecules and participate in reactions. In nonpolar solvents, oxygen can still donate a pair of electrons to form a bond with another atom or molecule.
Oxygen can act as a nucleophile in many different types of reactions, such as nucleophilic substitution, nucleophilic addition, and nucleophilic acyl substitution. Some common examples include the addition of water to an alkene to form an alcohol, the substitution of a leaving group with an alcohol in an SN2 reaction, and the addition of a nucleophile to a carbonyl group in an acyl substitution reaction.