Arcarius
- 14
- 0
Homework Statement
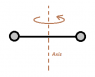
An oxygen molecule consists of two oxygen atoms whose total mass is 5.3x10^-26 kg and whose moment of inertia about an axis perpendicular to the line joining the two atoms, midway between them, is 1.9 x 10^-46 kg*m^2. From this data, estimate the effective distance between the atoms.
Homework Equations
## I = M1R^2 + M2R^2 ##
The Attempt at a Solution
I = (M1+M2)R^2
1.9 x 10^-46 kg*m^2 = (5.3 x 10^-26kg)R^2
R = 5.99 x 10^-11 m
Although I got that this is the radius, this is not the answer to the problem. I'm not exactly sure what I did wrong, as it seems right to me. Could anyone check my work? Thanks!