Carlos_Ishigami07
- 10
- 2
- TL;DR Summary
- How many seconds does it take for the paper to burn?
I have a problem: in the same anime I saw that Senku estimated the time to start burning the paper in sixty seconds, one minute and with a lens.
The data is as follows:
The lens he used has a diameter of 5 centimeters.
The solar constant, assuming it is the same as 3700 years ago, is 1362 watts per square meter.
The radiation index, or convection heat without wind, is zero.
The specific heat of paper is 1300 J / (kg * K)
The density of the paper is 900 kilograms per cubic meter.
These are the key equations:
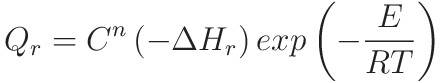
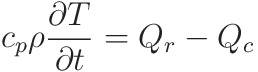
The data is as follows:
The lens he used has a diameter of 5 centimeters.
The solar constant, assuming it is the same as 3700 years ago, is 1362 watts per square meter.
The radiation index, or convection heat without wind, is zero.
The specific heat of paper is 1300 J / (kg * K)
The density of the paper is 900 kilograms per cubic meter.
These are the key equations:
Last edited by a moderator: