eliassiguenza
- 24
- 0
Homework Statement
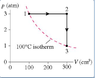
I have a diagram of a process of a monoatomic gas.
and the problem is that I know that the total amount of work = total amount of heat,
so there is no internal energy change. why? because my process started at the same temp that it finished. how ever... it is asking me the heat supplied from 1--->2 when they kept constant pressure, and 2----->4 when they kept constant volume
Homework Equations
W = nRT ln V2/V1
The Attempt at a Solution
please help me with this but do not give me the answer otherwise i won't learn! hehe
thank you!