nhrock3
- 403
- 0
this is the general way i solve every question:
example of a solved question in this way
we put 0.8 mole of H2 1.3 mole of I2 in one litter cantainer
the balance constant is 10.7
calculate how much 2HI will be in the container when the reaction balances
H2 +I2 = 2HI
start 0.8 1.3 0
moves -x -x +2x
end 0.8-x 1.3-x 2x
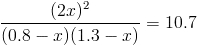
there are two solutions but only one is possiblr
x=0.0604
[HI]=0.12how to solve this question in the same way
we mix 0.5 moles of A
1 mole of B
2 moles of C
in a one litter container
after it balances we find 0.6 moles of A
find K for
=(is a sign where two arrows go in different directions)
2A =B+3c
example of a solved question in this way
we put 0.8 mole of H2 1.3 mole of I2 in one litter cantainer
the balance constant is 10.7
calculate how much 2HI will be in the container when the reaction balances
H2 +I2 = 2HI
start 0.8 1.3 0
moves -x -x +2x
end 0.8-x 1.3-x 2x
there are two solutions but only one is possiblr
x=0.0604
[HI]=0.12how to solve this question in the same way
we mix 0.5 moles of A
1 mole of B
2 moles of C
in a one litter container
after it balances we find 0.6 moles of A
find K for
=(is a sign where two arrows go in different directions)
2A =B+3c
Last edited: