gracy
- 2,486
- 83
Why 2-Bromopropane is called isopropyl bromide?I am concern about term iso.I have been told if there is one CH3 group as a substituent on the c atom next to end c atom then we use term iso but here in 2-Bromopropane
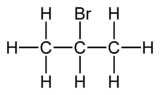
there is no ch3 group on the c atom next to end c atom one ch3 group does exist but it is not substituent.Infact I have been thinking this iso configuration as it is only applied to branched compounds otherwise it is we use n-...
and 2-Bromopropane is not branched at all ,although one halo group is present as substituent but carbon chain is not branched.
there is no ch3 group on the c atom next to end c atom one ch3 group does exist but it is not substituent.Infact I have been thinking this iso configuration as it is only applied to branched compounds otherwise it is we use n-...
and 2-Bromopropane is not branched at all ,although one halo group is present as substituent but carbon chain is not branched.