AnthonyCChem
- 1
- 0
In an isobaric expansion process, the pressure of the gas is kept constant. Because of the increase in volume the constant pressure can only be archived by increasing temperature. Then I want to ask is the pressure of the gas necessarily equal to the external pressure throughout the whole process?
It is because many times when we see a P-V diagram is plotted as a horizontal line then we say the process is isobaric. Is it right at the first place? The Pressure on the y-axis should be the pressure of the gas rather than the external pressure. However when calculating work for such a horizontal line we use w=-Pex delta V and substitute the P of the y-axis to the equation while the y-axis should represent pressure of the gas.
It is what I understand. If the P-V diagram is a horizontal line it just tell us the pressure of the gas is unchanged while expanding. Calculating work require us to know the pressure which stop the gas from expanding but instead we use the pressure of the gas to do the calculation. Does that mean the pressure of the gas is equal to that of the external pressure? If not I think there is a lack of information to calculate work for such process as we don't know the external pressure the gas go against.
The next is something I want to ask when calculating work.
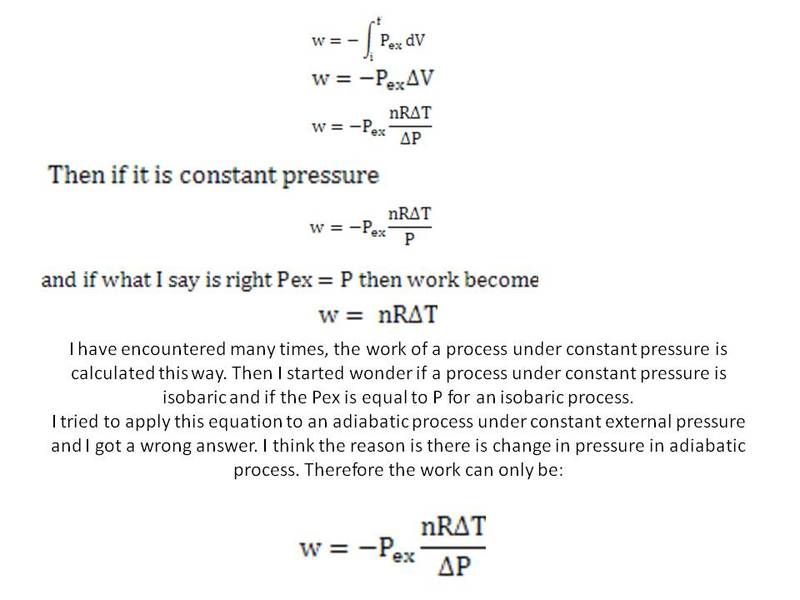
Also I would like to ask about free expansion. In free expansion the work done by the perfect gas against the vacuum is zero. The final temperature of the gas remain unchanged so delta U is zero and q is zero too. That means the gas does not lose any energy through heat and work. Then why the expansion would stop? Would the gas expand to a point which the pressure of the gas equal zero to stop because it is expanding against vacuum? If the pressure of the gas is not zero then the gas has not established equilibrium as one side there is pressure while on the other there is no pressure.
Desperate for answer.
It is because many times when we see a P-V diagram is plotted as a horizontal line then we say the process is isobaric. Is it right at the first place? The Pressure on the y-axis should be the pressure of the gas rather than the external pressure. However when calculating work for such a horizontal line we use w=-Pex delta V and substitute the P of the y-axis to the equation while the y-axis should represent pressure of the gas.
It is what I understand. If the P-V diagram is a horizontal line it just tell us the pressure of the gas is unchanged while expanding. Calculating work require us to know the pressure which stop the gas from expanding but instead we use the pressure of the gas to do the calculation. Does that mean the pressure of the gas is equal to that of the external pressure? If not I think there is a lack of information to calculate work for such process as we don't know the external pressure the gas go against.
The next is something I want to ask when calculating work.
Also I would like to ask about free expansion. In free expansion the work done by the perfect gas against the vacuum is zero. The final temperature of the gas remain unchanged so delta U is zero and q is zero too. That means the gas does not lose any energy through heat and work. Then why the expansion would stop? Would the gas expand to a point which the pressure of the gas equal zero to stop because it is expanding against vacuum? If the pressure of the gas is not zero then the gas has not established equilibrium as one side there is pressure while on the other there is no pressure.
Desperate for answer.
Last edited: