Technon
- 17
- 3
I'm thinking about the wave–particle duality and I like to understand it through analogy. So I'm thinking about how it relates to for example water. Water can be seen as both having wave characteristics, but also as particles (water molecules). Do you think this is a good analogy to the wave–particle duality of quantum physics? Give arguments why and/or why not you think so.
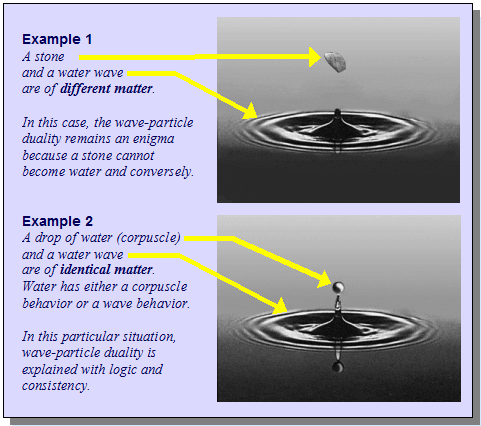
(Note: The picture is not mine, I just searched "wave particle duality water" and it came up, and I thought it relates somewhat to my question).
(Note: The picture is not mine, I just searched "wave particle duality water" and it came up, and I thought it relates somewhat to my question).
