mubashirmansoor
- 258
- 0
I have been wondering how to realize the color of a mineral by having the absorption Spectra...
The following case is an example;
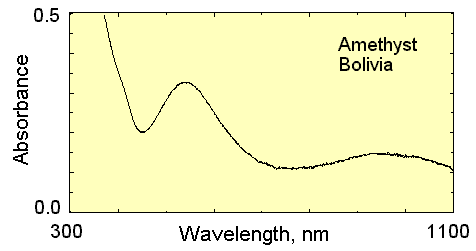
The image is the absorption spectra of a Bolivian Amethyst which looks like this;
[PLAIN]http://www.tequilabay.com/amethyst300lbbolivian.jpg
I have tried interpreting the purplish color just by reading the spectra, but I wasn't successful... So I will be very thankful for your guidance.
The following case is an example;
The image is the absorption spectra of a Bolivian Amethyst which looks like this;
[PLAIN]http://www.tequilabay.com/amethyst300lbbolivian.jpg
I have tried interpreting the purplish color just by reading the spectra, but I wasn't successful... So I will be very thankful for your guidance.
Last edited by a moderator: