Discussion Overview
The discussion revolves around understanding how the absorption spectra of minerals, specifically a Bolivian Amethyst, relates to their perceived color. Participants explore the relationship between absorption peaks and the resulting color, considering both theoretical and practical aspects of light interaction with materials.
Discussion Character
- Exploratory
- Technical explanation
- Conceptual clarification
- Debate/contested
Main Points Raised
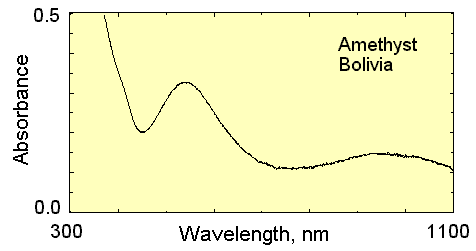
- One participant questions how to interpret the color of a mineral using its absorption spectra, specifically mentioning the purplish color of the Bolivian Amethyst.
- Another participant notes that absorption below 400 nm is UV and suggests focusing on the absorption peak around 500-600 nm, indicating that the complementary color to green-yellow is purple.
- A different participant explains that when light interacts with an object, it can be absorbed, transmitted, or reflected, and emphasizes that reflection is what is perceived as color.
- One participant seeks clarification on how to quantify the exact tone or wavelength of color from the absorption data, expressing uncertainty about the broader absorption spectrum from 450 to 700 nm.
- Another participant asserts that the graph provides the only quantitative description of color, arguing that perceived color encompasses all absorbed wavelengths rather than a single wavelength.
- One participant draws an analogy between perceived color and taste, suggesting that both involve multiple components at varying intensities.
- A later reply suggests combining the absorption graph with a light source graph and integrating the results using "tristimulus values" for human vision.
Areas of Agreement / Disagreement
Participants express differing views on how to interpret the absorption spectra in relation to perceived color, with no consensus on the best method for quantifying color tone from the data.
Contextual Notes
Participants highlight the complexity of color perception, noting that it involves multiple wavelengths and intensities, and that the interpretation of absorption spectra may depend on various assumptions and definitions.