- #1
warenzeichen
- 20
- 0
Quoting from wikipedia
I understand what symmetrical and antisymmetrical stretching come from and the number of vibrational mode(s) can be predicted basing on the concept of degree of freedom.
However, for scissoring, rocking, wagging and twisting, are they considered as bending modes/motion? Can CO2 undergo all these four kinds of motion?
From the notes and resources, I can only figure out the in-plane and out-of-plane bending of CO2, but no scissoring,rocking, wagging and twisting? or are they just the subset of so-called bending modes?
Thanks.
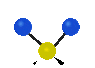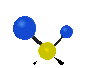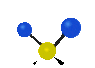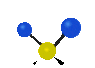
the atoms in a CH2 group, commonly found in organic compounds can vibrate in six different ways: symmetrical and antisymmetrical stretching, scissoring, rocking, wagging and twisting:
Symmetrical
stretching
I understand what symmetrical and antisymmetrical stretching come from and the number of vibrational mode(s) can be predicted basing on the concept of degree of freedom.
However, for scissoring, rocking, wagging and twisting, are they considered as bending modes/motion? Can CO2 undergo all these four kinds of motion?
From the notes and resources, I can only figure out the in-plane and out-of-plane bending of CO2, but no scissoring,rocking, wagging and twisting? or are they just the subset of so-called bending modes?
Thanks.