MichaelLAndersonEE
- 2
- 0
I am not an electrochemist, but I do need to understand various issues with an analytical chemistry instrument. This is a somewhat open-ended question.
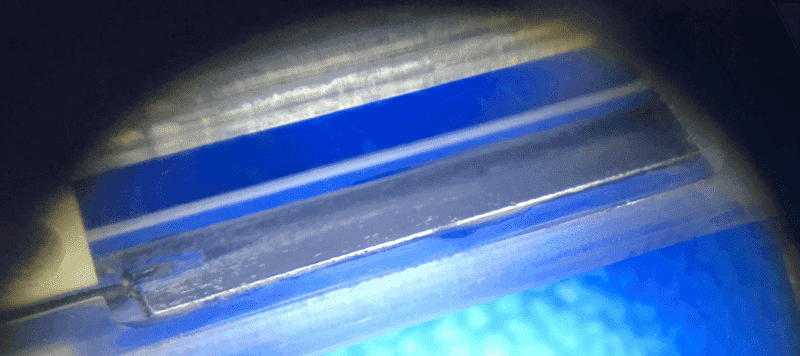
The instrument applies a voltage waveform across two palladium electrodes. Typical waveform is 2 Hz, 4 Vpk sinusoid, for brief intervals. There may be a slight, erroneous DC component. Plate separation is 3 to 5 mm. Plate measures 5 x 18 mm. The media vary widely, but a standard one is 1 mM KCl. pH can vary widely, but 7.0 is my baseline. We measure conductance and other properties of the media this way.
One of the biggest questions concerns the palladium electrodes. They perform very differently when they are polished clean versus coated with a deposit. I really need to understand this better, since it is by far the biggest factor in reaching accurate measurements.
What is the deposit? We do not know. My guess is 'palladium black', possibly palladium chloride. It is a thin, dark gray coating, easily rubbed off with a swab. The electrode housing is typically PMMA and polystyrene (for neutral pHs and moderate temps, of course).
There are two effects of concern here. 1.) The measured conductance depends upon the exposed plate area. I may be mistaken, but this need not be strictly area normal to the opposing electrode. In other words, the entire wetted surface is what is communicating with the ions, inducing their motion. The structure of the palladium coating (which I understand to be quite peculiar) may be adding to this effective area? Is this a fractal question? 2.) I need to know the E field between the plates, but only in the centermost 1 mm. Regardless of their surface irregularities, the E field should be textbook parallel in the center, yes? If not, why not? Of course there will be fringing at the edges, but how would the coating affect that? And even if it did, edge fringing should not affect the center 1 mm volume that I care about.
How do I make the deposit? I run the waveform for about 20 minutes with a solution of 1.3 Molar NaCl. The medium then looks dingy brown. I wash the electrodes thoroughly and then they yield measurements within 7% tolerance versus a standard. If I use polished electrodes my error is more like 20%, always lower in magnitude. This is not a random error. Effectively, with the polished electrodes my E field is diminished by the corresponding amount. The palladium deposit not only enhances the ion mobility, but in a way that is fundamentally necessary to the experiment.
Other interesting questions pertain to double layer formation and to osmotic flow, especially if there is a small DC bias to the waveform.
Suggestions of course most welcome, any reference texts or articles are equally valuable.
The instrument applies a voltage waveform across two palladium electrodes. Typical waveform is 2 Hz, 4 Vpk sinusoid, for brief intervals. There may be a slight, erroneous DC component. Plate separation is 3 to 5 mm. Plate measures 5 x 18 mm. The media vary widely, but a standard one is 1 mM KCl. pH can vary widely, but 7.0 is my baseline. We measure conductance and other properties of the media this way.
One of the biggest questions concerns the palladium electrodes. They perform very differently when they are polished clean versus coated with a deposit. I really need to understand this better, since it is by far the biggest factor in reaching accurate measurements.
What is the deposit? We do not know. My guess is 'palladium black', possibly palladium chloride. It is a thin, dark gray coating, easily rubbed off with a swab. The electrode housing is typically PMMA and polystyrene (for neutral pHs and moderate temps, of course).
There are two effects of concern here. 1.) The measured conductance depends upon the exposed plate area. I may be mistaken, but this need not be strictly area normal to the opposing electrode. In other words, the entire wetted surface is what is communicating with the ions, inducing their motion. The structure of the palladium coating (which I understand to be quite peculiar) may be adding to this effective area? Is this a fractal question? 2.) I need to know the E field between the plates, but only in the centermost 1 mm. Regardless of their surface irregularities, the E field should be textbook parallel in the center, yes? If not, why not? Of course there will be fringing at the edges, but how would the coating affect that? And even if it did, edge fringing should not affect the center 1 mm volume that I care about.
How do I make the deposit? I run the waveform for about 20 minutes with a solution of 1.3 Molar NaCl. The medium then looks dingy brown. I wash the electrodes thoroughly and then they yield measurements within 7% tolerance versus a standard. If I use polished electrodes my error is more like 20%, always lower in magnitude. This is not a random error. Effectively, with the polished electrodes my E field is diminished by the corresponding amount. The palladium deposit not only enhances the ion mobility, but in a way that is fundamentally necessary to the experiment.
Other interesting questions pertain to double layer formation and to osmotic flow, especially if there is a small DC bias to the waveform.
Suggestions of course most welcome, any reference texts or articles are equally valuable.