Discussion Overview
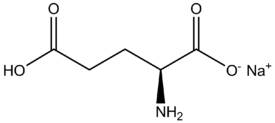
The discussion revolves around the process by which glutamic acid loses a hydrogen ion to form an ionic bond with sodium, specifically in the context of acid-base reactions and the terminology associated with this process. Participants explore concepts related to oxidation, reduction, dissociation, and the driving forces behind these reactions.
Discussion Character
- Conceptual clarification
- Debate/contested
- Chemical reasoning
Main Points Raised
- One participant questions whether the loss of hydrogen in glutamic acid is termed "oxidized" and seeks clarification on the process that allows for the formation of an ionic bond.
- Another participant references acid-base reactions that produce salts, providing an example of a reaction and noting its exothermic nature as a driving force.
- A third participant introduces the term "dissociation" as relevant to the discussion.
- One participant asserts that losing a hydrogen cation is always a reduction, arguing that the hydrogen leaves without taking electrons, thus increasing the electron count for the molecule.
- Another participant challenges the reduction claim, stating that in the case of strong acids, there is no charge transfer, and dissociation should not be classified as reduction or oxidation, suggesting that this reasoning could incorrectly categorize other dissociation reactions.
Areas of Agreement / Disagreement
Participants express differing views on the classification of the loss of hydrogen as oxidation or reduction, indicating a lack of consensus on the terminology and underlying principles involved in the reaction.
Contextual Notes
The discussion includes unresolved definitions and assumptions regarding oxidation, reduction, and dissociation, as well as the specific conditions under which these terms apply.