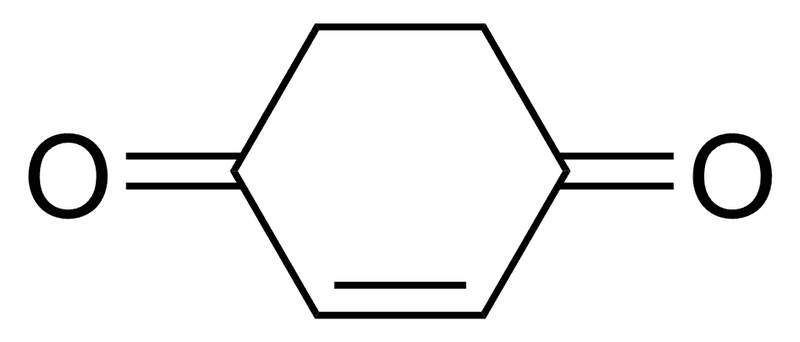
The discussion centers on the percentage of the enol form of a compound in relation to its keto form, with participants debating whether it is 99% or 1%. The consensus leans towards the enol form being 1% due to the stability of the keto form, which is aromatic and thus more stable. One participant highlights the role of Gibbs free energy in determining the equilibrium between the two forms, noting that small energy differences can lead to significant variations in composition. Aromatic stabilization energies, estimated between 20 to 40 kcal/mol, further support the dominance of the keto form in this tautomeric equilibrium. The conversation also touches on the importance of understanding aromaticity in this context, with a request for drawings of the enolic form to clarify its structure.