rwooduk
- 757
- 59
This may seem a bit pedantic but I'm writing a report and have radicals in it and notice that some papers put the dot in different places, so my question is does it matter where you put the dot?
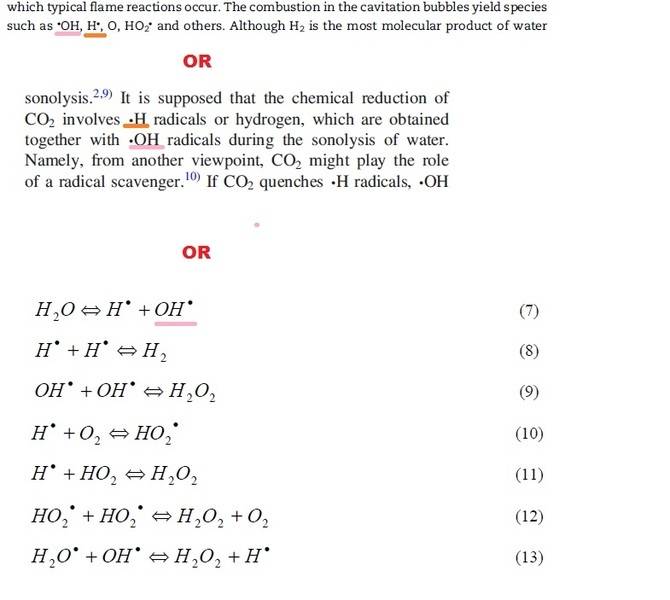
thanks for any advice
thanks for any advice