Discussion Overview
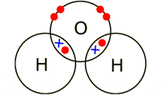
The discussion revolves around the correct representation of water molecules using covalent dot and cross diagrams, focusing on the validity of different drawing conventions and their implications for understanding molecular structure. Participants explore the theoretical and pedagogical aspects of these representations.
Discussion Character
- Conceptual clarification
- Debate/contested
- Meta-discussion
Main Points Raised
- Some participants note that diagrams are representations and do not have direct physical meaning, emphasizing that students often misinterpret these models as literal truths.
- There is a suggestion that there is no single "proper way" to draw these diagrams; rather, there are conventions that can be chosen based on context, such as the course textbook used.
- One participant highlights the importance of understanding the underlying logic of the diagrams rather than treating the conventions as facts.
- Another participant expresses gratitude for the input received and acknowledges their own limited knowledge, indicating a desire for further understanding.
- A participant recommends consulting introductory texts on theoretical chemistry for deeper insights into the topic.
Areas of Agreement / Disagreement
Participants express differing views on the existence of a "proper way" to draw molecular diagrams, with some arguing that conventions are flexible while others emphasize the importance of adhering to educational standards. The discussion remains unresolved regarding the best practices for teaching these representations.
Contextual Notes
Participants acknowledge that the diagrams are simplifications of reality and that the logic behind them is more significant than the specific conventions used. There is an emphasis on the need for clarity in teaching these concepts to students.