aphyx
- 11
- 0
Hey!
I am looking into how batteries work but I can't understand why -- from a chemical perspective -- voltage increases when they are connected in series.
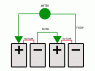
Let's say we have two identical batteries: battery 1 at the bottom and battery 2 on top, connected in series. The negative bottom end of battery 1 connects to the positive top end of battery 2 via a wire. The positive top end of battery 1 connects to the negative bottom end of battery 2 directly.
From what I've read about battery cells, there is a chemical separator in the middle of each battery that prevents electrons to flow directly from one side of the battery to the other. If that's true, then the bottom end of battery 1 and the top end of battery 2 (the only parts of the batteries that connect to the wire) are completely isolated from the direct exchange of electrons that happens at the place where the two batteries connect. Thus, I conclude that in effect, there is only one battery powering the wire, consisting of the bottom end of battery 1 which shares electrons with the top end of battery 2.
Why is voltage increased when in reality there are only two half-cells powering the circuit?
Thank you!
I am looking into how batteries work but I can't understand why -- from a chemical perspective -- voltage increases when they are connected in series.
Let's say we have two identical batteries: battery 1 at the bottom and battery 2 on top, connected in series. The negative bottom end of battery 1 connects to the positive top end of battery 2 via a wire. The positive top end of battery 1 connects to the negative bottom end of battery 2 directly.
From what I've read about battery cells, there is a chemical separator in the middle of each battery that prevents electrons to flow directly from one side of the battery to the other. If that's true, then the bottom end of battery 1 and the top end of battery 2 (the only parts of the batteries that connect to the wire) are completely isolated from the direct exchange of electrons that happens at the place where the two batteries connect. Thus, I conclude that in effect, there is only one battery powering the wire, consisting of the bottom end of battery 1 which shares electrons with the top end of battery 2.
Why is voltage increased when in reality there are only two half-cells powering the circuit?
Thank you!