SUMMARY
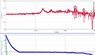
The discussion centers on the phenomenon of voltage generation during the freezing of water, specifically measuring fluctuations in voltage using a Vernier LabQuest and probes. Voltage readings ranged from 0.400 V to -0.200 V, attributed to potential fractures in ice or galvanic reactions. The participants emphasized the importance of using identical materials for probes to eliminate galvanic effects and suggested that the presence of ions in water is crucial for voltage generation. The conversation highlights the complexity of measuring induced voltages in both fresh and saltwater environments.
PREREQUISITES
- Understanding of electromechanical phenomena in ice
- Familiarity with galvanic reactions and their implications
- Knowledge of voltage measurement techniques using multimeters
- Basic principles of water chemistry, including ion concentration
NEXT STEPS
- Research "Electromechanical Phenomena in Ice" for deeper insights into voltage generation
- Explore methods to measure galvanic reactions in water using identical metal probes
- Investigate the effects of ion concentration on voltage generation in fresh and saltwater
- Learn about the use of temperature differentials in measuring induced voltages in ice
USEFUL FOR
Researchers, physics students, and anyone interested in the electrical properties of water and ice, particularly in experimental settings involving voltage measurement during phase changes.