Happiness
- 686
- 30
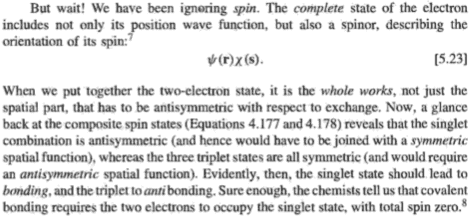
The book explains covalent bonding is due to exchange forces of attraction, which isn't a real force but the last term in [5.22]. This term arises due to electrons being indistinguishable particles.
If electrons were distinguishable, there would be no exchange forces. Then, would there still be covalent bonds? In other words, are there other factors that give rise to a covalent bond? Would the Coulombic forces of attraction between distinguishable protons and distinguishable electrons produce a covalent bond? Is it that exchange forces just make a covalent bond stronger or is it that without them, there will be no covalent bonds?
Are covalent bonds evidence that electrons are indistinguishable particles?
Reference: Intro to QM, David J Griffiths, p208
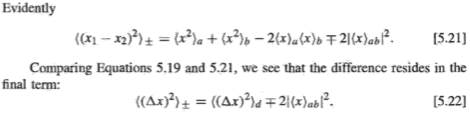

If electrons were distinguishable, there would be no exchange forces. Then, would there still be covalent bonds? In other words, are there other factors that give rise to a covalent bond? Would the Coulombic forces of attraction between distinguishable protons and distinguishable electrons produce a covalent bond? Is it that exchange forces just make a covalent bond stronger or is it that without them, there will be no covalent bonds?
Are covalent bonds evidence that electrons are indistinguishable particles?
Reference: Intro to QM, David J Griffiths, p208