gpavanb
- 11
- 0
Homework Statement
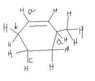
Look at the diagram accompanying the question. Which has least C-H bond energy?
The Attempt at a Solution
On cleavage of a C-H bond, a carbanion is formed as carbon is more electronegative than hydrogen. So cleavage of (b) results in a carbanion stabilized by resonance