Thermodinamics Definition and 41 Threads
-
N
Undergrad The missing 3D magnetism theory
If 2D magnetism (flat magnets, QM magnetism) exhibits perfect symmetry, following Maxwell’s equations, but 3D magnetism transforms most repulsive interactions by torque into attraction, with spherical bodies being impossible to repel, if Modern Physics says “the fact that 3D magnetism repulsion...- nataliaeggers
- Thread
- Electomagnetism Thermodinamics
- Replies: 6
- Forum: Classical Physics
-

Undergrad Entropy and configurations of microstates
I was watching a Khan Academy video on entropy called: Reconciling thermodynamic and state definitions of entropy. So in the video it says: Let's say I have a container. And in that container, I have gas particles and they're bouncing around like gas particles tend to do, creating some pressure...- lost captain
- Thread
- Entropy Permutation Statisical physics Thermodinamics
- Replies: 5
- Forum: Thermodynamics
-

How to calculate a coefficient of performance (COP)
Homework Statement Homework Equations [/B][/B] 3. The Attempt at a Solution Here's my solution attempt: NOTE: Evolution 2 to 3 is really an isothermic process, but in the diagram shows as an politropic process. 4. Further Questions But it isn't giving me logical values of COP...- Screwed
- Thread
- Coefficient Cop Cycle performance Thermo Thermodinamics
- Replies: 12
- Forum: Introductory Physics Homework Help
-
G
Volume ratio in an adiabatic gas expansion
Homework Statement Consider a perfect monoatomic gas at pressure $p_i$ 1.2 atm and temperature $T_i$ 300K, that is in equilibrium inside a cylinder having a volume $V_i=1L$ and which piston has a mass of 1kg and is at an height of 50 cm. Admit that a mass M=3.13kg is over the piston. When that...- Granger
- Thread
- Adiabatic Expansion Gas Gas expansion Piston Piston cylinder Ratio Thermodinamics Volume
- Replies: 9
- Forum: Introductory Physics Homework Help
-
S
Thermodynamics Hydrogen question
1.The problem statement: Hydrogen (H2) is at standard conditions in a closed tank, V = 5l, and then it's cooled down for 55K. Find delta U and Q. 2. Questions: If it says standard conditions, can I just use pV = nRT to find n and then deltaU = j/2*nR(delta)T? Also, is there any work that a gas...- smiljanic997
- Thread
- Hydrogen Thermodinamics Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
H
Experimental thermodynamics properties
Hello everybody, I am using an equation of state to calculate the thermodynamical properties of a material. I have a problem which is the ideal part of the equation. Actually, I cannot be sure that the ideal contribution in my equation is the really ideal contribution, so I decided to calculate...- hosein
- Thread
- Experiemnt Experimental Properties Thermodinamics Thermodynamics
- Replies: 1
- Forum: Chemistry
-

Rankine Cycle Efficiency Calculation - Superheated Steam at 40 bar and 500°C
Homework Statement Superheated steam at a pressure of 40 bar and a temperature of 500°C is supplied to the turbine of a Rankine cycle. If the condenser pressure is 0.03 bar. Find the thermal efficiency of the cycle. (Neglect feed pump work). I used steam tables found...- MCTachyon
- Thread
- Cycle Efficiency Rankine cycle Thermodinamics
- Replies: 8
- Forum: Engineering and Comp Sci Homework Help
-

Calc Volumetric Flow Rate of Free Air: 0.841 m3/s
Homework Statement Air is to be compressed from a pressure of 1 bar and a temperature of 20°C to a pressure of 15 bar in a two stage compressor with intercooling. After intercooling the air temperature returns to 20°C and the polytropic index of compression is 1.28. If the input power per...- MCTachyon
- Thread
- Air Air compressor Compressor Thermodinamics Volumetric flow rate
- Replies: 41
- Forum: Engineering and Comp Sci Homework Help
-
V
Thermodynamics, bullet melting ice problem
Homework Statement An ice cube at the melting temperature that has a mass of 20 g, is struck by a bullet with a mass of 9 g, flying at a certain speed. Determine the speed of the bullet, if it is known that one third of his energy was consumed to break the ice, and the remainder to melt it...- Vitalius6189
- Thread
- Bullet Ice Melting Thermodinamics Thermodynamics
- Replies: 4
- Forum: Introductory Physics Homework Help
-
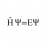
Finding compressibility from given internal Energy function
Hi everyone! 1. Homework Statement Given is a function for the internal energy: ##U(T,V)=Vu(T)## Asked is to derive the entropy balance equation. In order to do so i need to find the "isothermal and adiabatic compressibility": $$\kappa_{T}=-\frac{1}{V}\left(\frac{\partial V}{\partial...- H Psi equal E Psi
- Thread
- Compressibility Energy Entropy Function Internal Internal energy Thermodinamics
- Replies: 7
- Forum: Advanced Physics Homework Help
-
G
Graduate Negative T for a spin 1 system in the canonical ensemble
I'm interested in an apparent inconsistency with the result for negative temperatures for a spin 1 system of N particles. The partition function of such a system is \begin{equation} Z=(1+2\cosh(\beta \,\epsilon))^{N} \end{equation} where each particle can be in one of three energy states...- Gabriel Maia
- Thread
- Canonical ensemble Ensemble Negative Spin System Thermodinamics
- Replies: 1
- Forum: Thermodynamics
-
T
Undergrad Do laws of thermodynamics really apply to ecosystems?
From Odum (Father of modern ecology) Ecosystem follows the laws of termodynamics 1st law 2nd law The way the laws have been put it looks alright but they are valid for closed systems only which ecosystems are not. However according to wikipedia apart from having a closed system there are...- Tyto alba
- Thread
- Apply Biophysics Laws Thermodinamics Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-
Graduate Entropy: Thermodynamics & Information Theory Explained
I was reading some articles related to entropy and I come to know that, The term “Entropy” shows up both in thermodynamics and information theory. Now my question is : What’s the relationship between entropy in the information-theory sense and the thermodynamics sense? I need some clear and...- Jam Smith
- Thread
- Entropy Term Thermodinamics
- Replies: 6
- Forum: Thermodynamics
-
D
Should I learn fluid mechanics or thermodynamics
I am graduate student from structural mechanics of solids and need to learn 1 course from fluids and thermal group which includes fluid mechanics or thermodynamics+heat transfer. I am unsure which one would be better to learn, especially which would be helpful in future for solid mechanics. Can...- dsf356
- Thread
- Fluid Fluid dynamics Fluid machanics Fluid mechanics Mechanics Solid mechanics Structural mechanics Thermodinamics Thermodynamics
- Replies: 1
- Forum: STEM Academic Advising
-
B
How to read a Thermodynamic table?
When solving a word problem in Thermodynamics and are given both pressure and tempreature of a fluid or water, how do we know if we should get information about internal energy and entropy from the Pressure Table or the Temperature Table?- Beembo
- Thread
- Table Thermodinamics Thermodynamic
- Replies: 2
- Forum: Mechanical Engineering
-
H
Undergrad How to determine temperature of filament?
I was wondering in class today how I could determine the temperature of a filament in an incandescent light bulb? Mainly then so I could determine the thermal energy outputted through radiation. So after school I used a test circuit. I started with the Stefan-Boltzmann law which is Q =...- HHeCNeOSiFe
- Thread
- Circuit Filament Temperature Thermodinamics
- Replies: 1
- Forum: Thermodynamics
-

System of particles with non-degenerate energy levels
Homework Statement A system has three non-degenerate energy levels with energies 0, ε, and 2ε. a) Calculate the entropy of the system if the three levels are populated by two distinguishable particles such that the total energy is U=2ε. b) Calculate the entropy of the system if the three...- Elvis 123456789
- Thread
- Energy Energy levels Levels Particles Statistical mechanics System System of particles Thermodinamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Biosystems Eng.: pasteurization system for peanut butter
Homework Statement In recent years, there have been several significant outbreaks and recalls associated with Salmonella in peanut butter. One company has designed a thermal pasteurization system to reduce pathogen risk in peanut butter, in which the peanut butter is pumped through a heating...- A Furious Potato
- Thread
- Biological Biomedical engineering System Thermodinamics
- Replies: 3
- Forum: Engineering and Comp Sci Homework Help
-
W
Why Does the Position of Psat(P) Change in Kp,eq Formula?
Homework Statement question1-sentence https://www.physicsforums.com/attachments/105257 question1-answer https://www.physicsforums.com/attachments/105258 question2-sentence https://www.physicsforums.com/attachments/105259 question2-answer https://www.physicsforums.com/attachments/105260...- williamcarter
- Thread
- Constant Equilibrium Equilibrium constant Thermodinamics
- Replies: 20
- Forum: Biology and Chemistry Homework Help
-

Graduate Thermodynamics : https://www.youtube.com/watch?v=uc9P5yb3Xtc
Hi I had a question about this video. Indeed if I understand the video correctly it states that energy is linked to information. But how does that work indeed knowing that energy is constant, that more entropy = less information and that entropy increases. It seems that the universe will end...- TheAnt
- Thread
- Thermodinamics Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-
I
High School Conservation of energy in quantum physics
I am still in secondary school so I probably shouldn't think about things this complicated (at least that's what it seems to me, complicated), but please correct me if I'm wrong. If I recall correctly, the position of an electron is never certain, and always based on probability, unless...- IAmJustCurious
- Thread
- Atomic Conservation Conservation of energy Energy Physics Quantum Quantum phyics Quantum physics Thermodinamics
- Replies: 5
- Forum: Quantum Physics
-
The Pressure of a liquid in equilibrium?
The pressure of a liquid in equilibrium, is equal to the pressure of it's vapour, or to the sum of vapour pressure plus atmospheric pressure? My doubt starts from this problem: At 293 K and 1 atm, the vapour pressure of water is 565.8 Pa. Calculate the vapour pressure of water, when the total...- Eureka99
- Thread
- Equilibrium Liquid Pressure Thermodinamics
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
S
Graduate Internal energy in irr. process with molar heat not constant
I'm a bit confused about the following situation. In a irreversible thermodynamics process the molar heat of an ideal gas changes according to a function of the temperature, say ##c_v=f(T)## (which also leads to ##c_p=R+f(T)##) and I'm asked to determine the heat exchanged during that process...- Soren4
- Thread
- Constant Energy Heat Internal Internal energy Isochoric Process Temperature Thermodinamics
- Replies: 2
- Forum: Thermodynamics
-

Undergrad How Do Carnot's Efficiency Formulas for Heat Engines Align?
My question is: according to Carnot cycle, the maximum efficiency of a heat engine is given by 1 - T2/T1, where T2 is the temperature of the cold source and T1 the temperature of the hot source. So, accordingly, as higher T2 is for a same T1, lowest is the efficiency of the engine. But, the...- Tulio Cesar
- Thread
- Carnot cycle Carnot engine Engines Heat Heat engine Heat engines Thermodinamics Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-
T
Temperature after mixing ice and water
Homework Statement 5 g of water at 30°C and 5 g of ice at -20°C are mixed together in a calorimeter Find the final temperature of the mixture. Water equivalent of the calorimeter is negligible,specific heat of ice=0.5 cal/g°C and latent heat of ice =80 cal/g. Homework Equations...- takando12
- Thread
- Calorimetry Ice Mixing Temperature Thermodinamics Water
- Replies: 4
- Forum: Introductory Physics Homework Help
-
T
How Many Times Can the Piston Operate Per Air Bottle in a Pneumatic Lift System?
Homework Statement A pneumatic lift system is being demonstrated at a sales show. The total load is 70 kg, and the lift piston is 15.2 cm in diameter and has a 20.2 cm stroke. A portable air bottle with an initial pressure of 20 MPa and a temperature of 23 Celsius degrees is to be used as the...- TonyBoyle
- Thread
- Lift Pneumatic Thermodinamics
- Replies: 7
- Forum: Engineering and Comp Sci Homework Help
-
L
Graduate Why does heat engine need to do negative work to surround?
Every thermodynamics cycle needs to do negative work to the environment, which lower its total positive work. For example, in Carnot cycle, the most efficiency possible: 1/ Engine receives heat from hot reservoir, expands and do positive work to surround 2/ Surround does work to engine...- lemd
- Thread
- Engine Heat Heat engine Negative Thermodinamics Work
- Replies: 12
- Forum: Thermodynamics
-
C
Heat conduction and phase changes
Homework Statement Suppose we have a lake and a layer of ice on top such that the bottom of the lake is maintained at a constant temperature T_{bot} which is above the freezing point of water, and top of the ice is maintained at the air temperature T_{air} which is below the freezing point of...- ClassicalMechanist
- Thread
- Conduction Heat Heat and thermodynamics Heat conduction Phase Thermodinamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
A
Graduate Confusion With Blackbody Radiation
A blackbody is a theoretical object that perfectly absorbs all the light that falls on it. From what I understand this is an ideal situation and does not actually exist in reality. Certain objects are close to being a blackbody but they do not absorb 100% of the light that hits it (i.e. some...- Amanda5455
- Thread
- Blackbody Blackbody radiation Confusion Radiation Terminology Thermodinamics
- Replies: 1
- Forum: Thermodynamics
-
H
Graduate Example of entropy as function of temperature only
Hi, I know that changes in entropy can be expressed as a function of temperature, specific volume, and pressure using the fundamental equations of thermodynamics: ds = du/T+pdv/T, where the changes in entropy can be caused by either changing the specific volume or the internal energy. I also...- hoomanya
- Thread
- Entropy Example Function Internal energy Temperature Thermodinamics
- Replies: 3
- Forum: Thermodynamics
-
F
Release or absortion of energy on bonds and phase change
I believe I can explain why there is energy needed to break intermoleculares bonds and getting into a gas or liquid, but the other way around confuses me. Bonds have potential energy associated to it, so It's needed work to break the bonds, because we would be trying to move a molecule away from... -
M
Engineering heat transfer problem for critical radius
Homework Statement A pipe of internal radius r1=0.03 m is built to have conductivity variable with radius: k=ar2, where a=250 Wm/K. 1) Find the critical radius r2 for the maximum heat transfer by convection from its external surface if the heat transfer coefficient is h2=30 W/m2K. Homework...- mostar
- Thread
- Engineering Heat Heat and mass transfer Heat transfer Radius Thermodinamics
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
F
Undergrad What determines if a reaction is exotermic or endotermic?
Using a more physics oriented approach, rather than a chemistry one, and looking to an energy point of view if possible. For example, why when ice turns into water there is a need for energy (heat)? I know in some extent that the energy is used into re-organizing the chemical bonds, but that...- FG_313
- Thread
- Chemistry Conservation of energy Heat Reaction Thermodinamics
- Replies: 4
- Forum: Atomic and Condensed Matter
-
A
Calculating work heat, and efficiency given a TS diagram
Homework Statement Is it possible to calculate the work done heat transfer, and efficiency of an object in a thermodynamic system, given the Temperature vs Entropy graph? Example: Homework Equations ∆U=Q-W ∆S=dQ/T e=1-(Qc/Qh) e=W/QhThe Attempt at a Solution Since this is a T vs S diagram,i...- abobo37
- Thread
- Diagram Efficiency Entropy Heat Internal energy Thermodinamics Work
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-

Turbocharger: Mass flow rate and pressure ratio
Hello everyone, i am currently dimensioning a compression system using a vehicle turbocharger and need to find a way to relate the pressure ratio of the compressor and the mass flow rate. (flows trough compressor and turbine are of course separated) For the turbine, one can use Stodola's...- Carlko26
- Thread
- Compressor Flow Flow rate Mass Mass flow Mass flow rate Pressure Rate Ratio Thermodinamics Turbine Turbocharger
- Replies: 1
- Forum: Mechanical Engineering
-

Mixing of two air flow at different pressure
Hi Friends! Here is my query : I have two source of air supply with different pressures, temperatures and with different mass flow rates. Input 1: P1,T1,m1. Input 2:P2,T2,m2. Output: P3,T3,m3. P,T and m refers to pressure, temperature and mass flow rate respectively. If the mixing is being done...- Devang Marvania
- Thread
- Adiabatic Air Air flow Flow Mixing Pressure Thermodinamics
- Replies: 2
- Forum: Mechanical Engineering
-
A
Graduate A brief question on quantum thermodinamics.
Hi, I am new to this forum so I don't really know if this question already exists.. My question is: When an electron absorbs a foton and climbs to the next energy gap and then returns again, as the energy is quantum energy, how is possible that the foton reemited by the electron has the same...- Alex Cros
- Thread
- Quantum Thermodinamics
- Replies: 8
- Forum: Quantum Physics
-
T
Graduate Statistical Thermodinamics: how many ways to make a set of population?
Hi everyone! Here's my problem of the day: Let's take a box containing 3 identical (but distinguishable) particles A B C. Let this be a canonical ensamble. Suppose that A has energy \varepsilon_0 and both B and C have energy \varepsilon_1. We thereforre have 2 energy level, n_0,n_1...- teddd
- Thread
- population Set Statistical Thermodinamics
- Replies: 1
- Forum: Thermodynamics
-
Z
Need help understanding the thermodinamics implications of an intercooler
Hello everyone, I am a car entusiast who likes to turn wrenches and modify things, but I like to do it first by understanding the teory behind each one of my projects. Here's the case study I would like to get some help on: The car is a BMW Z3 with a 4 cylinder 1.9 liters engine. Few...- ZetaTre
- Thread
- Thermodinamics
- Replies: 1
- Forum: Mechanical Engineering
-
C
Another thermodinamics exercise,
The volume of a cylinder, with adiabatic walls and closed at the ends, has been divided in two parts by an adiabatic plate (with unimportant volume), which can slide without friction inside the cylinder (it is like a movable piston). The cylinder has been filled with an ideal diatomic gas...- claudiadeluca
- Thread
- Exercise Thermodinamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
How Do You Calculate Temperature and Energy Changes in Mixed Ideal Gases?
I tried but I cannot do it. Please help. Two rigid adiabatic containers have inside the same ideal gas, respectively N1=3mol at a temperature T1=340K and N2=2mol at a temperature T2=280K. The two containers come into contact by opening a faucet, and the gas mixes, getting to a temperature T3...- claudiadeluca
- Thread
- Exercise Thermodinamics
- Replies: 1
- Forum: Introductory Physics Homework Help